A Single-Stage Hyaluronic Acid Matrix for Cartilage Repair
Hyalofast
Hyalofast® is a biodegradable, non-woven scaffold composed of HYAFF® (benzyl ester of hyaluronic acid) intended as support for the entrapment of mesenchymal stem cells (MSC) for the repair of chondral and osteochondral lesions. It acts as a support for bone marrow aspirate (BMA), bone marrow aspirate concentrate (BMAC), or as a chondroprotective coverage which favors in situ residence of mesenchymal stem cells after their mobilization following bone marrow stimulation procedures.
Hyalofast fibers favor cell adhesion, allowing MSC arrangement inside the three-dimensional matrix. Once implanted, it fills the defect and temporarily substitutes the chondral and osteochondral tissue in the lesion until it is absorbed and replaced by the cartilage tissue. As the HYAFF fibers degrade, the polymer releases the parent molecule enriching the implantation site with hyaluronic acid, a naturally occurring extracellular matrix molecule and a major component of human cartilage.
The unique handling properties of Hyalofast make it quickly and easily implantable via arthroscopy or mini-arthrotomy. The soft texture allows it to conform easily to the lesion anatomy. It readily adheres to the site of application without requiring additional fixation in most cases. The scaffold can be applied in any orientation or stacked due to its uniform single-layer 3D structure.
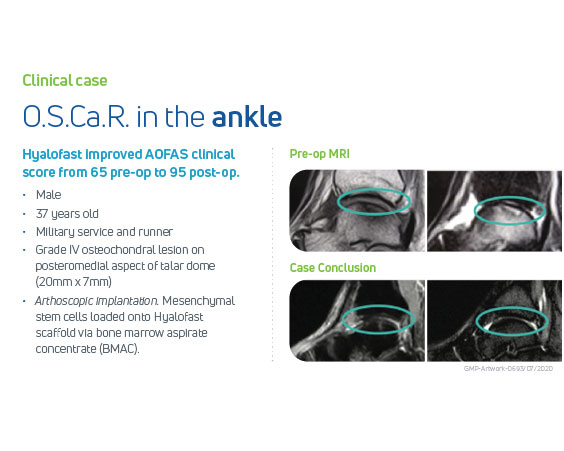
Hyalofast has been clinically demonstrated to be safe and effective in medium-to-long-term follow-up of 8 years in the repair of ankle, knee or hip chondral and osteochondral lesions, permitting the filling of the lesion with a high volume of new hyaline-like cartilage tissue, and smooth integration with the surrounding cartilage. Patients reported reduction in pain and good functional improvement of the affected injured joint, thus enabling return to their daily/non-impact sport activities with substantial pain relief and high satisfaction.
Key Features & Benefits:
- Single-stage, off-the-shelf cartilage repair procedure
- Versatile: acts as a scaffold for bone marrow aspirate or as a chondroprotective coverage after bone marrow stimulation procedures; usable either in open or minimally invasive surgery
- Fast: can be used with or without fixation means; easily adaptable to any lesion shape
- Two available sizes allow different lesion sizes to be easily covered: 2×2 cm and 5×5 cm
- Clinically demonstrated to be safe and effective up to medium-long-term follow-up of 8 years
Hyalofast is not approved for use in the United States.
Hyalofast is CE-marked indicated for the repair of chondral and osteochondral lesions.
Anika products may not be available in all geographies. Product availability is subject to the regulatory clearances in individual markets. Please reach out to your local representative or Contact Us if you have questions about specific market approvals.
For complete product information, including indications, contraindications, warnings, and precautions, please refer to the Instructions for Use provided with the product or Contact Us for a copy.
Click here to visit the Hyalofast website:
Hyalofast website- Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res. 2009 Dec;467 (12):3307-20. Epub 2009 May 16.
- Buda R, Vannini F, Cavallo M, Grigolo B, Cenacchi A, Giannini S. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Joint Surg Am. 2010 Dec;92 Suppl 2:2-11.
- Battaglia M, Rimondi E, Monti C, Guaraldi F, Sant’Andrea A, Buda R, Cavallo M, Giannini S, Vannini F. Validity of T2 mapping in characterization of the regeneration tissue by bone marrow derived cell transplantation in osteochondral lesions of the ankle. Eur J Radiol. 2011 Nov;80(2):e132-9. Epub 2010 Aug 30.
- Vannini F, Battaglia M, Buda R, Cavallo M, Giannini S. “One step” treatment of juvenile osteochondritis dissecans in the knee: clinical results and T2 mapping characterisation. Orthop Clin North Am. 2012; 43(2):237-44
- Gobbi A., Chaurasia S., Karnatzikos G., Nakamura N. Matrix-Induced Autologous Chondrocyte Implantation versus Multipotent Stem Cells for the Treatment of Large Patellofemoral Chondral Lesions: A Nonrandomized Prospective Trial. Cartilage 2015, Vol. 6(2) 82– 97.
- Buda R., Vannini F., Castagnini F., Cavallo M., Ruffilli A., Ramponi L., Pagliazzi G., Giannini S. Regenerative treatment in osteochondral lesions of the talus: autologous chondrocyte implantation versus one-step bone marrow derived cells transplantation. Int Orthop. 2015 May; 39(5):893-900. Epub 2015 Feb 8.
- Rafols C, Amenabar T, Monckeberg Diaz J E, Huamani C, Numair J. Severe Chondral Injuries Associated to FAI Treated with HYALOFAST Scaffold, Microfractures and Peripheral Blood Stem Cells. Clinical and Imaging Results with a Minimum Follow-Up of 12 Months. J Hip Preserv Surg (2016) 3 (suppl_1).
- Gobbi A, Scotti C, Karnatzikos G, Mudhigere A, Castro M, Peretti GM. One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years. Knee Surg Sports Traumatol Arthrosc. 2017 Aug; 25(8):2494-2501. Epub 2016 Jan 14.
- Gobbi A, Whyte GP. One-Stage Cartilage Repair Using a Hyaluronic Acid-Based Scaffold With Activated Bone Marrow-Derived Mesenchymal Stem Cells Compared With Microfracture: Five-Year Follow-up. Am J Sports Med. 2016 Nov;44(11):2846-2854. Epub 2016 Jul 29.
- Sofu H, Kockara N, Oner A, Camurcu Y, Issın A, Sahin V. Results of Hyaluronic Acid-Based Cell-Free Scaffold Application in Combination With Microfracture for the Treatment of Osteochondral Lesions of the Knee: 2-Year Comparative Study. Arthroscopy. 2017 Jan;33(1):209-216. Epub 2016 Sep 7.
- Tahta M, Akkaya M, Gursoy S, Isik C, Bozkurt M. Arthroscopic treatment of osteochondral lesions of the talus: Nanofracture versus hyaluronic acid-based cell-free scaffold with concentration of autologous bone marrow aspirate. J Orthop Surg (Hong Kong). 2017 May-Aug; 25(2)
- Sofu H, Camurcu Y, Ucpunar H, Ozcan S, Yurten H, Sahin V. Clinical and radiographic outcomes of chitosan-glycerol phosphate/blood implant are similar with hyaluronic acid-based cell-free scaffold in the treatment of focal osteochondral lesions of the knee joint. Knee Surg Sports Traumatol Arthrosc. 2019 Mar;27(3):773-781. Epub 2018 Aug 1.
- Gobbi A, Whyte GP. Long-term Clinical Outcomes of One-Stage Cartilage Repair in the Knee With Hyaluronic Acid-Based Scaffold Embedded With Mesenchymal Stem Cells Sourced From Bone Marrow Aspirate Concentrate. Am J Sports Med. 2019 Jun;47(7):1621-1628. Epub 2019 May 16.
- Buda R, Baldassarri M, Perazzo L, Ghinelli D, Pagliazzi G. A useful combination for the treatment of patellofemoral chondral lesions: realignment procedure plus mesenchymal stem cell-retrospective analysis and clinical results at 48 months of follow-up. Eur J Orthop Surg Traumatol. 2019 Feb;29(2):461-470. Epub 2018 Sep 17.
- Yontar NS, Aslan L, Can A, Ogut T. One step treatment of talus osteochondral lesions with microfracture and cell free hyaluronic acid based scaffold combination. Acta Orthop Traumatol Turc. 2019 Sep;53(5):372-375. Epub 2019 May 21.
- Tan SI, Tho SJW, Tho KS. MF Biological resurfacing of grade IV articular cartilage ulcers in knee joint with HYALOFAST. J Orthop Surg (Hong Kong). 2020 Jan-Apr;28(1)
- Farr S, Pallamar M, Eder T, Ganger R. Treatment of advanced stage osteochondrosis dissecans in the adolescent elbow using a hyaloronic acid-based scaffold: a case series of 5 patients. Arch Orthop Trauma Surg. 2021 Feb 4. Online ahead of print.
- Bajuri MY, Sabri S, Mazli N, Sarifulnizam FA, Mohd Apandi H. Osteochondral Injury of the Talus Treated With Cell-Free Hyaluronic Acid-Based Scaffold (Hyalofast®) – A Reliable Solution. Cureus. 2021;13(9):e17928. Published 2021 Sep 13.
- Geyer S, Mattes J, Petersen W, Imhoff AB, Achtnich AE. Arthroscopic one-step matrix-assisted bone marrow stimulation for the treatment of osteochondral defects of the talus. Oper Orthop Traumatol. 2021 Oct 5. English. doi: 10.1007/s00064-021-00737-4. Epub ahead of print.